TEOS / Oxygen Thermal CVD
TEOS is tetra-ethyl-ortho-silicate, or equivalently tetra-ethoxy-silane:
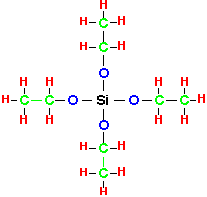
TEOS is a liquid at room temperature, with a vapor pressure of about 1.5 Torr. TEOS slowly hydrolyzes into silicon dioxide and ethanol when in contact with ambient moisture, but its flammability and toxicity are similar to that of an alcohol. To produce the vapor for use in processing, one may use a bubbler or a liquid injection system. In both cases, temperatures above room temperature are usually used to increase the TEOS partial pressure; thus it becomes necessary to heat the gas lines to prevent condensation therein. If a bubbler is used, it is important to ensure that the carrier gas is free of moisture; otherwise the slow accumulation of polymerized products in the TEOS reservoir will cause a decrease in vapor pressure and drift in process characteristics. For low chamber pressures (<10 Torr), the vapor over warm TEOS liquid can be metered directly through a heated low-pressure mass-flow controller.
The key to understanding the difference between TEOS and silane is to note that in TEOS the silicon atom is already oxidized: the conversion of TEOS to silicon dioxide is essentially a rearrangement rather than an oxidation reaction, with much reduced changes in free enthalpy and free energy.
Deposition
The basic overall reaction for the deposition of silicon dioxide requires the removal of two oxygen atoms:
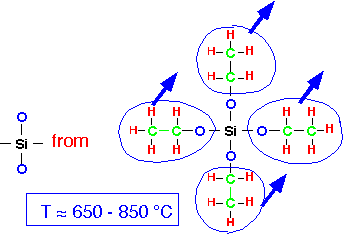
While gas phase reactions can occur, particularly at the high end of the temperature range, deposition is probably the result of TEOS surface reactions. TEOS chemisorbs onto silanol groups (Si-OH) at the surface, as well as strained surface bonds:
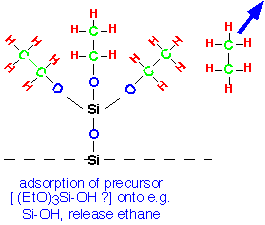
TEOS will not adsorb onto the resulting alkyl-covered surface, so deposition is probably limited by removal of the surface alkyl groups. These groups can undergo elimination reactions with neighboring molecules to form Si-O-Si bridges:
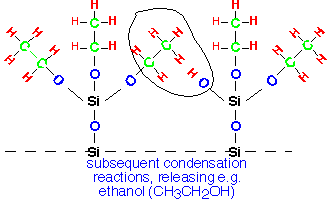
This process proceeds in an inert atmosphere: TEOS can be its own oxygen source, and SiO2 can be deposited from TEOS in nitrogen. However, addition of oxygen increases the deposition rate, presumably through providing an alternative path for removal of the ethyl groups from the surface. TEOS/O2 is generally performed in tube reactors at pressures of a few Torr; a mini-batch reactor for in situ flow of BPSG using this process was sold in the early 90's but is no longer available.
Consequences of Chemistry
Conformity and gap fill: Conformality is excellent under most conditions: the depositing species are inert and can diffuse readily into trenches and holes.
Smooth film: Relatively little gas phase reaction gives smooth films.
Low hydrogen: For high deposition temperatures (>650 °C) the film structure is densified during deposition, so very little Si-OH is incorporated in the film, and Si-H is not observed at all due the very strong Si-O bonds. Films are thus dense and fairly stable as deposited with modest compressive stress.
Limited applications: Reasonable rates are only obtained at temperatures > 600 °C, so that high temperature TEOS/O2 cannot be used after metal is deposited.
A few representative references:
"Model studies of dielectric thin film growth: Chemical vapor deposition of SiO2" J. Crowell, L. Tedder, H. Cho, F. Cascarano and M. Logan (UCSD/Lam) J Vac Sci Technol A8 1864 (1990)
"Mechanistic studies of dielectric thin film growth by low pressure chemical vapor deposition: The reaction of tetraethoxysilane with SiO2 surfaces" L. Tedder, G. Lu and J. Crowell (UCSD) J Appl Phys 69 7037 (1991)
"Two precursor model for low-pressure chemical vapor deposition of silicon dioxide from tetraethylorthosilicate" M. IslamRaja, C. Chang, J. McVittie, M. Cappelli, and K. Saraswat [Stanford] J. Vac. Sci. Technol. B11 720 (1993)
"Properties of silicon dioxide films prepared by low-pressure chemical vapor deposition from tetraethylorthosilicate" S. Rojas, A. Modelli, W. Wu, A Borghesi, and B. Pivac (SGS Thomson/U Pavia) J. Vac. Sci. Technol. B8 1177 (1990)
"LPCVD of Borophosphosilicate Glass from Organic Reactants" D. Williams and E. Dein (AT&T Bell Labs) J Electrochem Soc 134 657 (1988)
"Modulation-doped silicate glass deposited by low-pressure chemical vapor depostiion", M. Ilg, M. Kraxenberger, K. Uram, N. Sandler, C. Parks, S. Nguyen, Superlattices & Microstructures 24 #5 p. 385
Return to Tutorial Table of Contents
Book version of the CVD Tutorial
